Masayo Takahashi (高橋 政代) is a pioneer in stem cell-based clinical trial work and more specifically induced pluripotent stem cell (IPSC)-based trials. Her new clinical study is another groundbreaking step in that it is organoid based, in this case for Retinitis pigmentosa.
This post is an informative Q&A interview with Dr. Takahashi on her new organoid clinical study. You can see an image below from their foundational mouse transplant work. Ref provided for researchers to this pub as well.
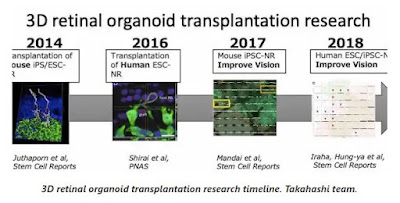
Then later in the post Paul has included a timeline that Dr. Takahashi sent of the efforts and she noted that sub-team leader Dr. Michiko Mandai MD, PhD is doing a great deal of this work.
PK: This is the trial in the world to use a IPS cell derivative for Retinitis pigmentosa, correct? What preclinical work went into the foundation for this?
MT: Yes. First transplantation utilize organoids and first obvious reconstruction of CNS.
Some past related work on retinal differentiation and organoids from pluripotent cells are below:
- Assawachananont et al Stem Cell Reports 2014
- Fujii et al Sci Rep., 2016
- Mandai et al Stem Cell Reports, 2017
- Iraha and Tu et al Stem Cell reports, 2018
- Tu and Watanabe et al EbioMedicine, 2019
- Akiba et al. eCollection 2019
PK: What is the rationale for using a retinal organoid as opposed to a sheet or suspension of retinal cells?
MT: We will use tiny retinal progenitor cell sheets cut from retinal organids.
For more than 10 years from the 2006 Nature paper, it had been believed that cell suspension transplant works well in the retina. But it was proved to be cytoplasmic transfer in 2016 or so.
We believed from the beginning that tissue transplantation is necessary to reconstruct the photoreceptor layer. Our papers were right.
PK: Are there other cells present in the organoids besides photoreceptors? Does the purity matter? What organoid protocol is being used and how long are the organoids allowed to develop?
MT: We will transplant organoids that consists of only retinal progenitors. It will maturate in the patients eye for several months to generate mature photoreceptors. There are other types of retinal cells, but still it is OK to recover the visual function in the completely photoreceptor degenerated retinal
PK: How will the organoids be deployed? Are they surgical implanted in the retina? How organoids used per eye?
MT: Cut tiny sheets from the organdies and transplant into the subretinal space.
PK: Do you predict the organoids will integrate into the eye and the cellular and neural network?
MT: We vigorously confirmed this for nearly 10 years using electrophysiology and behavior tests, which you can see in our papers.
PK: How many patients will be transplanted and how long will the follow-up be?
MT: The plan is 2 patients to start and 1 year of observation. A clinical trial is planned by a company after this study.
Source: The Niche
Masayo Takahashi (高橋 政代) is a pioneer in stem cell-based clinical trial work and more specifically induced pluripotent stem cell (IPSC)-based trials. Her new clinical study is another groundbreaking step in that it is organoid based, in this case for Retinitis pigmentosa.
This post is an informative Q&A interview with Dr. Takahashi on her new organoid clinical study. You can see an image below from their foundational mouse transplant work. Ref provided for researchers to this pub as well.
Then later in the post Paul has included a timeline that Dr. Takahashi sent of the efforts and she noted that sub-team leader Dr. Michiko Mandai MD, PhD is doing a great deal of this work.
PK: This is the trial in the world to use a IPS cell derivative for Retinitis pigmentosa, correct? What preclinical work went into the foundation for this?
MT: Yes. First transplantation utilize organoids and first obvious reconstruction of CNS.
Some past related work on retinal differentiation and organoids from pluripotent cells are below:
- Assawachananont et al Stem Cell Reports 2014
- Fujii et al Sci Rep., 2016
- Mandai et al Stem Cell Reports, 2017
- Iraha and Tu et al Stem Cell reports, 2018
- Tu and Watanabe et al EbioMedicine, 2019
- Akiba et al. eCollection 2019
PK: What is the rationale for using a retinal organoid as opposed to a sheet or suspension of retinal cells?
MT: We will use tiny retinal progenitor cell sheets cut from retinal organids.
For more than 10 years from the 2006 Nature paper, it had been believed that cell suspension transplant works well in the retina. But it was proved to be cytoplasmic transfer in 2016 or so.
We believed from the beginning that tissue transplantation is necessary to reconstruct the photoreceptor layer. Our papers were right.
PK: Are there other cells present in the organoids besides photoreceptors? Does the purity matter? What organoid protocol is being used and how long are the organoids allowed to develop?
MT: We will transplant organoids that consists of only retinal progenitors. It will maturate in the patients eye for several months to generate mature photoreceptors. There are other types of retinal cells, but still it is OK to recover the visual function in the completely photoreceptor degenerated retinal
PK: How will the organoids be deployed? Are they surgical implanted in the retina? How organoids used per eye?
MT: Cut tiny sheets from the organdies and transplant into the subretinal space.
PK: Do you predict the organoids will integrate into the eye and the cellular and neural network?
MT: We vigorously confirmed this for nearly 10 years using electrophysiology and behavior tests, which you can see in our papers.
PK: How many patients will be transplanted and how long will the follow-up be?
MT: The plan is 2 patients to start and 1 year of observation. A clinical trial is planned by a company after this study.
Source: The Niche