Ocata was bought by Astellas Pharma in February of 2016 and has since come under the wing of the Japanese pharmaceutical company to further it's leading research and development activities in regenerative medicine.
Dr. Lanza refers below to the change of name from Ocata to Astellas Institute for Regenerative Medicine ("AIRM").
I wrote a Blog post on the acquisition news entitled Science Validation & The Rising Sun.
Cheers
EYE Program
Ocata's hESC RPE Cell Therapeutic Candidate Review for Alexey Bersenev @ Stem Cell Assays
Product candidate name: hESC-MA09 RPE (no trade name yet)
Developer: Ocata Therapeutics Inc. (formerly Advanced Cell Technology Inc.)
History of development: Since 2003
Type of cells: Human allogeneic embryonic stem cell-derived retinal pigment epithelium (RPE), expanded ex vivo.
Tissue source: Blastomere cell derived from fertilized oocytes. Pre-embryo consent use provided as supernumerary tissue for cell extraction for science rather than discarding during standard IVF treatment. Patented non-embryo destructive technology (NED) used to establish hESC cell line – MA09.
Processing steps: Chemical ZP breaching, single blastomere cell extraction, isolated cell co-culture with pluripotent/hES cells in a Quinn’s Cleavage Medium, further culture steps using Quinn Blastocyst Medium on mitomycin C-treated MEF feeder layer with DMEM & bFGF. hESC master cell line established & banked. RPE spontaneous derivation following continued hES cell culturing methods, on MEF in medium without LIF, FGF and Plasmanate or from suspended embryoid body overgrowths in long term culture. The resulting pigmented islands of cells are enzymatically digested and RPE cells isolated and plated for differentiated primary cell cultures for passaging, expansion, working cell bank, harvest & cryopreservation.
Expansion: RPE population doublings ∼20
Phenotypic composition: Pax6-, bestrophin+, CRALBP+, PEDF+, RPE65+.
Exhibit a characteristic cobblestone, polygonal, epithelial-like appearance and comprise brown pigment dispersed within their cytoplasm.
Exhibit a characteristic cobblestone, polygonal, epithelial-like appearance and comprise brown pigment dispersed within their cytoplasm.
Stability/Safety: Genetically stable, normal karyotype, normal senescence & terminally differentiated to its RPE fate
Proposed mechanisms of action and potency: RPE cell replacement therapy aimed to restore lost retina cells at the base of the macula to establish a new and healthy support layer to the photoreceptors (Vitamin A, phagocytosis & waste processes, nutrients & factors). It is envisioned that by replacing the lost RPE layer the remaining photoreceptors will once again function properly thereby either arresting the decline of the age related condition or actually improve lost visual acuity.
Key publications:
- Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies (Lancet, 2014)
- Embryonic stem cell trials for macular degeneration: a preliminary report (Lancet, 2012)
- Long-term Safety and Function of RPE from Human Embryonic Stem Cells in Preclinical Models of Macular Degeneration (Stem Cells, 2009)
- Human Embryonic Stem Cell Lines Generated without Embryo Destruction – (Cell Stem Cell, 2007)
- Derivation of human embryonic stem cells from single blastomeres – (Nature Protocols, 2007)
(all publications are available for download on Ocata Therapeutics website)
Clinical Trials:
- Phase 1/2a USA – Age Related Macular Degeneration – Geographic Atrophy –NCT01344993
- Phase 1/2a USA – Stargardt’s Macular Dystrophy – NCT01345006
- Phase 1/2a UK – Stargardt’s Macular Dystrophy – NCT01469832
- Phase 1/2 Korea – Age Related Macular Degeneration – Geographic Atrophy –NCT01674829
- Phase 1/2 Korea – Stargardt’s Macular Dystrophy – NCT01625559
Administration: Vitrectomy with sub-retinal injection of cell suspension with dosing from 50,000 to 200,000 cells (Phase 1/2 design). Expected increase in ceiling for cell dose beyond 200,000 in Phase 2.
Key patents:
Blastomere hESC Culture System: – WO2006052646 & WO2007130664, US7838727 &US7893315 & US8642328 & US8742200 & US8796021, Espacenet Family
Stem Cell Derived RPE Cells: – WO2005070011 & WO2006080952 & WO2009051671 & WO2011063005 & WO2013074681, US7736896 & US7794704 & US7795025 &US8268303, Espacenet Family & Espacenet Family & Espacenet Family & Espacenet Family.
IP Positioning summary:
Ocata has arguably the leading clinical program for potentially curative cell therapeutics targeting AMD, SMD & MMD and other unmet medical conditions of the Eye. It’s IP in the area is considered robust and a growing “patent thicket” inhibitor to competitive segment entry using pluripotent cells. Dr. Paul Wotton, CEO of Ocata states that the company has an “Established IP position in major markets protecting the life span of the cell therapy – from the origin of the cell to the delivery into the patients’ eyes – hESC Blastomere, RPE Methods, Assays, Pharma Prep & Treatment Methods” Dr. Paul Wotton, CEO, Ocata Therapeutics, BioCEO N.Y. Feb. 2015.
There are two companies currently pursuing hESC RPE Dry AMD suspension products in the clinic – Cell Cure (a subsidiary of BioTime) and Ocata Therapeutics (previously Advanced Cell Technology). These two programs use different hESC source processes & RPE derivation methods to produce their RPE cell therapeutic product. Cell Cure’s product is from an hESC sourced from a blastocyst while Ocata’s product is sourced from an earlier pre-embryo state at the blastomere stage. Both groups differentiate their hESCs to a pure terminal RPE population. Cell Cure’s product uses a more chemically directed culture method which results in a stable RPE cell. Cell Cure’s program is almost 4 years behind Ocata’s lead.
In relation to Ocata’s hESC RPE suspension product therapy competition please see the following link: BTX Hadasit
Web Links, Related Posts & Misc. Media Articles:
Further, in order to provide further details of Ocata's lead program area focus, the Eye, I've provided the company's slides from a recent presentation, in which "Regenerative Ophthalmology" was highlighted.
Cheers
______________________________________________________
OCATA's Blood Program
The doctrine that we are all equal under God applies in reality to the very Blood of humanity that runs through our veins. This was the shocking truth that changed an age old way of intellectual & class division. After millennia Science did that not Faith. The fluid of life is an interchangeable commodity that drives the body and mind. A genetic brain unique to a person's cells is common enough to be of practical benefit. Donors of all types, colors and creeds can have some siphoned off and provided to those that need a refill.
We accept the DNA of another to live on, with new Blood, without question and without any harm.
With 7.2 Billion humans on the planet you would think that we'd have enough of the Red stuff to go around. Think again. We don't even have enough fresh supplies to meet current demand, let alone future requirements based on the donation system. How can we expect this system to suddenly change to meet the forecasted demand when the population hits 10 Billion within a few decades and then 15 Billion a generation or two later?
Enter science, as usual, to solve the human evolutionary dilemma - Create it.
Not only is it possible to do so now, in inexhaustible volumes to satisfy all, but those cell products can and will be modified by the Scientists & Doctors of this 2nd Blood Revolution to deliver the needed solutions against the parasites, funguses, viruses, bacterias et al. that plague & kill daily in countless numbers.
Engineered weapons of the vascular system that naturally hone in and destroy invading pathogens.
The below provides a rough summary of how this is coming together from the perspective of OCATA and it's scientific colleagues.
Cheers
Phase 1 - Blastomere Derived Renewable Stem Cell Line
Phase 2a - Clinical Expansion & Banking of Hemangioblast Derived Megakaryocytes
Phase 2b - Engineered Variants for Drug Delivery Requirements
Phase 3a - Differentiation of Platelets, Red Blood Cells & Line Derivatives
Phase 4a - Biocompatible Unit Preparation & Universal Distribution
Phase 4b - Locally Served Fresh Product via Bioreactor Automated Production
________________________________________
REFERENCES:
Cell Research Jan 2011 - "Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice" - SCRMI, Univ of Illinois Chicago, Cha Univ, Harvard / BWH & ACTC
Blood July 2014 - "Platelet bioreactor-on-a-chip" - Harvard / BWH, Univ Colorado & Colorado School of Mines, McGill, ACTC
Vector - Boston Children's Hospital Blog March 2014 - "The Platelet Whisperers"
MedCity News April 2014 - "Biochip mimics how the body produces platelets so they could be made in a lab"
NY Times May 2014 - "Young Blood May Hold Key to Reversing Aging"
UCSF July 2014 - "Key to Aging Immune System Is Discovered"
Proceedings of the National Academy of Sciences of the USA, June 2014 - Whitehead Institute & MIT - "Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes"
The Scientist Sept 2014 - "Next Generation: Blood-Cleansing Device" - (Engineered MBL Protein use example in fighting Sepsis - with cell engineering it can be done without dialysis)
Dr. Langer's Lab & Dr. Jensen's Lab of MIT
The Scientist July 2013 - "Narrow Straits - Transfecting molecules into cells is as easy as one, two, squeeze."
Proceedings of the National Academy of Sciences of the USA, Feb 2013 - "A vector-free microfluidic platform for intracellular delivery"
R&D, July 2013 - "Researchers put squeeze on cells to deliver"
Dr. Daley's Lab - "Hematopoiesis Research" - HHMI / Children's Hospital Boston / Havard & "CellNet" - Children's Hospital Boston / Havard / Boston Univ & iPS mRNA Tech
Daley / Children's Hospital Boston / Harvard Patent Families:
Biomechanical Induction of Hematopoiesis
Inhibition and Enhancement of Reprogramming by Chromatin Modifying Enzymes
Methods for Enhancing Hematopoietic Progenitor Cell Engraftment
Method to Produce Induced Pluripotent Stem (iPS) Cells from Non-Embryonic Human Cells
Method of Enhancing Proliferation and/or Hematopoietic Differentiation of Stem Cells
________________________________________
Advanced Cell Technology's Patent Portfolio for the Blood Program:
INEXHAUSTIBLE SOURCE OF RENEWABLE STEM CELLS:
Blastomere Non-Destructive Human Embryonic Stem Cell Technology
Blastomere Patent Family
5 Granted US Patents: 1, 2, 3, 4, 5
iPS/Reprogramming Renewable Stem Cell Derivation papers 1 & 2
iPS/Reprogramming Renewable Stem Cell Patent Family Portfolio: 1, 2 with examples A, B, C and SCNT Tech
EARLIEST YOUTHFUL BLOOD LINE POSSIBLE
HEMANGIO-COLONY FORMING CELLS - US Patent Granted & Patent Family
HEMANGIO COLONY FORMING CELLS AND NON-ENGRAFTING HEMANGIO CELLS - App Pub March 2011 & Patent Family
UNIQUE BLOOD LINE CELL PRODIGY DERIVATION:
(WO2011069127) LARGE SCALE GENERATION OF FUNCTIONAL MEGAKARYOCYTES AND PLATELETS FROM HUMAN EMBRYONIC
STEM CELLS UNDER STROMAL-FREE CONDITIONS - PCT Pub June 2011 & US App Pub Dec 2012 & Patent Family
(Note: SCRMI is a JV between ACTC & Cha Biotech of Korea - North American Rights belong to ACTC - Japan/Korea to Cha - ROW split)
(WO2014100779) METHODS FOR PRODUCTION OF PLATELETS FROM PLURIPOTENT STEM CELLS AND COMPOSITIONS THEREOF - PCT Pub June 2014 & US App Pub Sept 2014
______________________________________________________________
OCATA's Immune/Cancer Program - The Hemangioblast Progeny
When we speak of the various programs Ocata has in development, apart from the Eye, they are referred to as "other" and are separated into boxes so the general targets become clearer - Blood, Immune & Cancer.
However, it important to understand that all these "others," and their various derivative relations, are based off of the Hemangioblast cell state precursor... i.e. eImmune Modulation using MSCs, eCancer Targeting using Dendritic/NK cells & eBlood Repair using Platelets, all via Ocata's Non-Destructive hESC Platform source or from any of the alternative Pluripotent Platform technologies (i.e. iPS, Parthenogenesis, SCNT).
Tissue engineering (organs) isn't yet in the preclinical phase at Ocata but could of course be a focus of research and product development/IP licensing if the company opts to push forward in that direction down the line with Pluripotent cells, most likely with Autologous reprogrammed cells using iPS, Parthenogenesis or SCNT Pluripotent sources.
For now it's the Allogeneic Hemangioblast offspring and as such all discussions in Blood, MSC/Immune, Cancer should be considered in relation to this when reviewing what the opportunity is for product development.
A program for engineered eDendritic cells targeting a pathogenic or virus strain can be separated from an engineered eDendritic cell deal for a Cancer indication - both derived from a Patented Pluripotent eDendritic cell IP rights chain that goes back three Patent layers to source. However, on top of those source IP rights is the engineered cell product itself (synthetic Product Claim) which will be the Exclusive Product License.
Is it more beneficial to break-up IP than outright License it wholesale per cell type? Will this occur When and If unique synthetic versions can be developed to optimize & uniquely address target market indications? For example why License the entire Cancer market to one partner when there are multiple potential partners in the space? Granted a potential Partner may address a cross-section of the space but one Partner may only be interested in or dominate in a few disease areas while others may be active in unrelated diseases and so forth... Each can have a tailored product using exclusive IP from cell origin to intermediate cell states to final proprietary formulation...
This is the strategic design for securing multiple partnerships to Ocata's underlying Platform & Program IP.
It is the unique or synthetic nature of the final product for distinct targets that will exponentially and dramatically expand the scope of collaborative understanding with Industry players.
The explosion in awareness and movement in cellular targeting via synthetic biology is the beginning of the paradigm shift in medicine.
CAR-T has been a research focus for a couple of years ago now - which was a welcome adjunct to an on-again off-again research effort on immunity, genetics & gene editing technologies. The nature of beating disease by way of empowering & harnessing the immune/blood system is fundamental to next generation therapies. Emily was one such proof many had been hoping for. Not that pure cells themselves or synthetic editing aren't invaluable in their own right to fight disease, but that complex modified biological cell constructs are possible as future effective treatments.
As with all cell treatments the ability to manufacture an inexhaustible supply of a replicable standard as an off-the-shelf product is what will separate the mass market offering from the targeted niche. The cell progeny differentiated to a terminal state and used in therapeutics worldwide is akin to a small molecule drug produced on an assembly line.
Autologous treatments against Cancer may be required when specific and unique disease states & expression patterns apply and will therefore require bespoke cellular targeting. However, in the broader market a product with replicable standards, cost & scale will be vital and sought after by way of Allogeneic product methodologies.
Some Allo programs already exist based on adult cell sources however when the inherent expression benefits of Pluripotent derived cell factories are combined with synthetic multi-target gene edits and/or pathway modifications the final product becomes naturally more effective and valuable, in addition to the replicable conformity & economies of scale benefits.
Allogeneic product isn't by itself the definition of a complete solution purely because it's from an immortal source & is replicable to a standard but it is when combined with the inherently more potent properties of early cell state gene expression and enhanced synthetic action/targeting...
Product is the goal and when next gen product is available early prototypes, niche offerings & more costly general alternatives will be mooted.
Profitability models favor a wider adoption formula for acute, unmet and orphan diseases where there is no effective standard of care. Pricing will vary depending on volumes.
Repeat treatment requirements are yet to be determined in many cases for cellular therapies and may factor in to longer term chronic care maintenance, more in line with existing treatment options.
The science is on the cusp of having a solid fighting chance against disease, trauma & degeneration. That is perhaps something I wouldn't have said only a few years ago. However, doubt is gone in my mind and only time is left to answer what will be the optimal mechanism to defeat each target. Knowledge is expanding exponentially and as a result I imagine within a relatively short period of time the majority of our molecular pathways and stepwise complexities of action and reaction within our cellular communities will be known.
There is a race on that has only one possible ending - the complete and total banking of knowledge of the biological process map of our physical selves.
There is a race on that has only one possible ending - the complete and total banking of knowledge of the biological process map of our physical selves.
Just as The Human Genome Project opened the doors to a world of new discoveries, the uncovered realities of cellular based mechanisms are paving the way for fundamental changes in our societies that will impact the very nature of how we live our lives. Once disease is tamed our planet will turn to sustainability to maximize the efforts of our new longevity. The full cycle may take a generation or two to occur but it is inevitable and beginning now.
Ocata is one of the many at the forefront of this new dawn, contributing with knowledge & potential. Their unique understanding, developmental expertise, early cell state technology, IP positioning and mission is what sets them apart as a sector leader and underscores what the opportunity represents as a whole.
Cheers
______________________________________________________
OCATA's IP
The following two Ocata presentation slides detail the IP surrounding their Eye program.
As an early leader in the field of stem cell research Ocata is uniquely positioned with robust IP in all areas of Pluripotent Cell Sciences with notable Platform technologies in hESCs, iPS, SCNT & Parthenogenesis - please see Pluripotent Science tab.
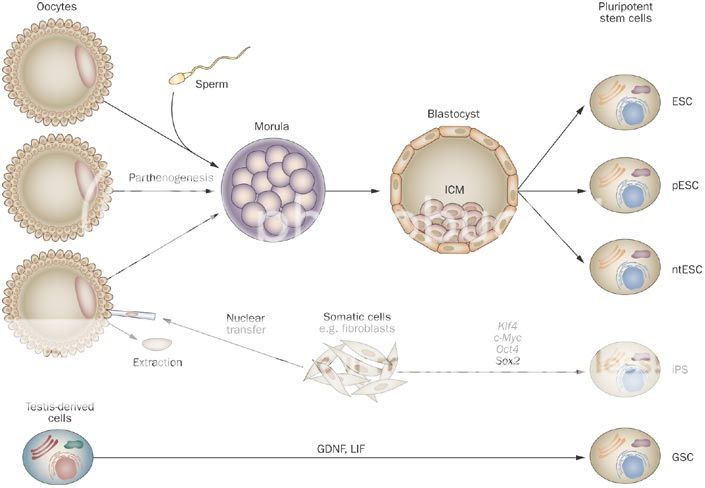
Ocata's Pluripotent Cell Sources
Stem cell science is inherently complex as it relates to biological systems which are only now becoming fully explored and understood. To many Stem Cell Therapeutics is new but in reality Bone Marrow (BM) transplants pioneered the sector over 40 years ago and the use of BM stem cells has been widely adopted throughout the world since then. Thus the sectors' Adult Stem Cells' origins led investigators to first develop more specific cell types for Adult Stem Cell Therapeutics, such as Adult Mesenchymal Stem Cells (MSCs) and terminal cell types of Adult MSC progenitor origin, such as Skin and Cartilage et al, which have been approved as the first purified and expanded Stem Cell Products available Internationally. To round out the background, importantly during this period James Thompson discovered a protocol to isolate, maintain and grow Human Embryonic Stem Cells (hESCs) in 1998 and the field opened up to the possibilities of using nature's master cells which are able to create any cell type in the body - i.e. a Pluripotent Cell - which Adult cells can't do. Also the potency and replicative power of these cells made hESC Therapeutics a viable cost effective medicinal option, which was therefore pursued by many in parallel to the work being done on Adult Stem Cells.
Now Pluripotent cells can be created from iPS and hESC technologies, as well as SCNT and Parthenogenesis. Adult Stem Cell researchers have also discovered recently a couple of potential sources of Pluripotent cells - Very Small Embryonic Like (VSELs) and Wisdom Tooth Dental Pulp (DPPSC) - and are investigating their capabilities. The nature of Pluripotency is important to grasp as the literature refers to Pluripotency as a general state, which also can be described as "embryonic" - however it doesn't necessarily mean derived from an Embryo, as the foregoing technologies prove. OCATA Therapeutics' (previously called Advanced Cell Technology - ACTC) historical work was to create "embryonic" cells by means of whatever technology was stable enough to generate viable Pluripotent cells... This work included extracting cells from an early stage fertilized human egg (human Embryonics - eventually Blastomere IP), from the false fertilization of a human egg, which cannot become a human (Parthenogenesis IP), from the transfer of an adult cell's nucleus to a human egg with fusion (SCNT or "Therapeutic Cloning" IP), from the reprogramming of an adult cell back in time to a Pluripotent state (De-differentiation IP - now called iPS)... Further to this OCATA was experimenting in those early days also with Trans-Differentiation, which is the reprogramming of an adult cell to directly become another adult cell type without reversing back to an intermediate Pluripotent state (Trans-differentiation IP). This early work was late 1990s to early 2000s...
As mentioned the term Pluripotency is by definition the ability to derive stem cells of any type. The Technology IP in the sector is how to create Pluripotent cells and then also how to maintain and differentiate those Pluripotent cells into the desired cell types and then scale those sufficient for Therapeutic marketing at a reasonable cost... As an analogy think of this as a Tree with a few Roots only that feed a Trunk - the Trunk is the Pluripotent state from which there emerges a number of Branches, which represent the Multipotent states, which then sprout Sub-Branches and then the leaf bearing Offshoots of those which produce the Leaves.... These Leaves are the finished individual cell types.. This is where the analogy breaks down somewhat as the Main Branches in the Tree, the Sub-Branches, the Offshoots and the Leaves would all be different colors representing different states along the unique path to the final cell types, nor does it represent the accurate number of possible intermediate step stages to get to the end result Cell Type - anyway you get the point I hope..
Human Embryonic Pluripotent Cells (hESCs) can be made by anyone soon given Thompson's WARF hESC derivation process is coming out of Patent Protection. That will open up hESC science to more innovation and programs - but it is a destructive process. In Europe the WARF Patents were cancelled and therefore the hESC research field was pursued with more interest than it was in the US. As everyone knows OCATA invented a unique approach to derive an hESC by way of a non-destructive process - Blastomere IP. That is what sets OCATA apart in getting to the Pluripotent Trunk stage using the embryo Root. No one else does that and they have a Patent in the US and Patent Applications submitted in other Territories to protect that hESC advantage. In Europe a non-destructive technology is required to pursue Patentability - OCATA awaits the European Legal process on its Blastomere Patent Application to secure its rights there. This is apart from the differentiation IP protocols of taking a Pluripotent cell and making it differentiate into a number of different Multipotent cell lineages and end cell types. These are what OCATA has a unique Priority based ability to develop and is pursuing broad Patent IP on for the Eye, Blood and Immune System areas... This positioning is unique in the world of hESC science for those areas of development IMO.
In respect to iPS Ocata are looking to secure the "Pluripotent" definition inclusion in Ocata's more recent Patent Extension Applications in all the above program areas as a right from the Original Filings and the inclusive Legal interpretation of the term "embryonic" in recent rulings. This has already been established in Australia for the Eye Program. The term iPS is a term coined by Yamanaka and as I relayed refers to taking an Adult Cell and converting it with reprogramming steps to a Pluripotent state - this is another of the Roots of the Tree Trunk. ACTC and others were doing this long before Yamanaka - OCATA's term for that was "De-differentiation" and the components of that technology are Patent Filings with v.early Priority Dates (late 1990s and early 2000s). A number of Patent Filings relate and ref to OCT4 as a differentiation / reprogramming protein used in the technological processes and methods that OCATA and others were experimenting with. In 2006 Yamanaka defined a set of 4 factors (incl. OCT4) that if used according to his protocol would convert Adult Cells back to a Pluripotent state. The fact that he documented and published this protocol in the Literature and made it widely available as a process for Lab Researchers made it a "standard" and won him the Nobel (rightly so). However, it doesn't negate the fact that other scientists had been working on the same reprogramming language, some way before Yamanaka (incl. OCATA). This is what Mgt. have been saying and that it has an early Priority on the use of OCT4 in the science. This of course relates to those using OCT4 in their protocols for iPS - however, it is an evolving field so the use of OCT4 in certain Protocols isn't necessary... I've stated my opinion on OCT4 in an iCell Thread.
Gary Rabin, then CEO of OCATA, relayed in an interview that OCATA's iPS approach was "zero-footprint reprogramming using vectorless technology" and is more to the point here as it's a reference to the difference in approaches being played out in the iPS field currently... Rather than getting caught up in defending the OCT4 Priority - which IMO Big Pharma partners will do later, if necessary - the current issue is how well do the various protocol approaches perform... That is still an open question but certainly I think most would agree now that an integration free route (i.e. not entering the genome) is safer - hence the effort to use steps that avoid integrating. However, the use of vectors (irrespective of Viral use or not) is also at issue here and it's been shown in certain cases to create residual effects. Yamanaka and Thompson use vectors. The leading safety protocols are the Harvard related iPS teams - Daley's mRNA process and Kim/OCATA's protein method... Safety first is the goal - hence the keen interest in Research Licenses for freely available integrating and non-integrating vector based iPS lines (Yamanaka and Thompson) but slow progress on Therapeutic Programs based on same... It is still too early to determine which Pluripotent route is best suited for mass market therapeutics but clearly OCATA is amongst the hand full of leaders in the field and one could say is the front runner with it's safety first iPS protocol pending IND submission selecting a denucleated cell type (Platelets) as a further safety precaution. In addition, OCATA has a potential lock on the Pluripotent RPE derivation that the other front runner, Yamanaka, is pursuing as his first indication, which would add distance to OCATA's first mover position...
In summary OCATA's Pluripotent strategy is to link this fundamental Trunk source for cell derivations lineages for the Eye, Blood and MSC Programs. Apart from that the Pluripotent protocols are valuable as stand alone Root Platforms - Blastomere, iPS Protein Reprogramming, SCNT and Parthenogenesis. These foundational Root technology approaches, plus Trans-Differentiation tech, make up the knowledge base and IP of the company generally and are interwoven as part of its Scientific Tool Kit. The breadth of the science IP protects the company from sector challenges - even if it's not using a part of the technology IP vault for a specific program.... Patents granted are more valuable than Applications of course but in this field knowledge and innovation are the keys to success given it's moving so quickly - a first mover advantage with IP is a powerful force.
To conclude it has been said that "Ocata have some of the best developmental biologists in the world" and it's clear they have IP in ALL Pluripotent technological avenues and are pioneering the science with a first mover strategy in ALL Root processes, which therefore suggests that if any one process is successfully translated this will establish OCATA's future and those associated.
Cheers
Root Pluripotent Processes:
This video below of Dr. Lanza's scientific presentation goes into detail about the various steps mentioned in the summary above - note that the Doctor talks about all Root IP approaches as part of his scientific knowledge base tool kit (hESC, SCNT, iPS and by default Parthenogenesis when referring to the Egg - see below Patent App).
The Cytoplasm is nature's incubator. What Ocata have here is a foundational approach to reprogram an old adult cell backwards in time to be youthful via a young new Egg, along the way using technology to modify.. This effectively is the road to healthy longevity via a natural cell regression mechanism that uses you're own adult cells in further steps to generate pluripotent cells for therapeutics... Previously OCATA needed access to a constant supply of human eggs and therefore it wasn't viable - now it is. SCNT is the term used for this as it stands for Somatic Cell Nuclear Transfer and is now to be combined with OCATA's iPS and Parthenogenesis Platforms using ES culturing differentiation technology for patient specific cells and tissues as an alternative route to deliver Cell Therapeutics. Genetic modifications are part of the overall process generally...
Effectively this states that OCATA uses Eggs to nurture regression, then uses SCNT technology or Parthenogenesis to derive "embryonic" cells for Therapeutic use... Before the lack of human Eggs was the bottleneck and the ethical issues associated with creating human embryos. With this technology approach we have choice and control over the entire process of developing Pluripotent cells without human fertilized embryos and without the possibility of viable embryo by products... iPS allows us to revert an Adult cell to become an Egg and with controlled stimulation "embryonic/Pluripotent" cells can be produced without any possibility of developing viable human embryos... This is Lanza's scientific stem cell tool kit brilliance and what makes OCATA unique in that it has ALL the necessary pieces of the puzzles and knows how to assemble them.
Further General References:
Embryonic stem cell - Wikipedia, the free encyclopedia
Induced pluripotent stem cell - Wikipedia, the free encyclopedia
Somatic-cell nuclear transfer - Wikipedia, the free encyclopedia
Parthenogenesis - Wikipedia, the free encyclopedia
Transdifferentiation - Wikipedia, the free encyclopedia
Adult stem cell - Wikipedia, the free encyclopedia (VSELs)
Added Dr. Zarbin's papers & presentation:
http://www.revophth.com/content/d/retina/c/37809/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176062/
http://www.asorn.org/client_data/fil...zarbin2012.pdf



.jpg)

.gif)