Stem cell science is inherently complex as it relates to biological systems which are only now becoming fully explored and understood. To many Stem Cell Therapeutics is new but in reality Bone Marrow (BM) transplants pioneered the sector over 40 years ago and the use of BM stem cells has been widely adopted throughout the world since then. Thus the sectors' Adult Stem Cells' origins led investigators to first develop more specific cell types for Adult Stem Cell Therapeutics, such as Adult Mesenchymal Stem Cells (MSCs) and terminal cell types of Adult MSC progenitor origin, such as Skin and Cartilage et al, which have been approved as the first purified and expanded Stem Cell Products available Internationally. To round out the background, importantly during this period James Thompson discovered a protocol to isolate, maintain and grow Human Embryonic Stem Cells (hESCs) in 1998 and the field opened up to the possibilities of using nature's master cells which are able to create any cell type in the body - i.e. a Pluripotent Cell - which Adult cells can't do. Also the potency and replicative power of these cells made hESC Therapeutics a viable cost effective medicinal option, which was therefore pursued by many in parallel to the work being done on Adult Stem Cells.
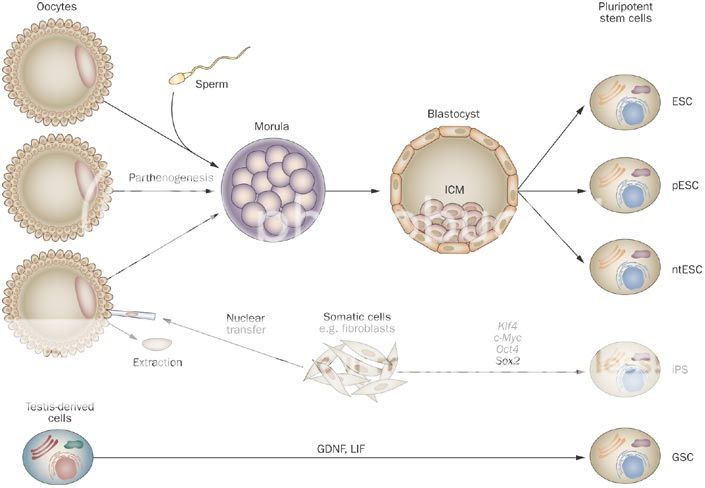
Now Pluripotent cells can be created from iPS and hESC technologies, as well as SCNT and Parthenogenesis. Adult Stem Cell researchers have also discovered recently a couple of potential sources of Pluripotent cells - Very Small Embryonic Like (VSELs) and Wisdom Tooth Dental Pulp (DPPSC) - and are investigating their capabilities. The nature of Pluripotency is important to grasp as the literature refers to Pluripotency as a general state, which also can be described as "embryonic" - however it doesn't necessarily mean derived from an Embryo, as the foregoing technologies prove. Ocata Therapeutics' (formerly Advanced Cell Technology "ACTC") historical work was to create "embryonic" cells by means of whatever technology was stable enough to generate viable Pluripotent cells... This work included extracting cells from an early stage fertilized human egg (human Embryonics - eventually Blastomere IP), from the false fertilization of a human egg, which cannot become a human (Parthenogenesis IP), from the transfer of an adult cell's nucleus to a human egg with fusion (SCNT or "Therapeutic Cloning" IP), from the reprogramming of an adult cell back in time to a Pluripotent state (De-differentiation IP - now called iPS)... Further to this OCATA was experimenting in those early days also with Trans-Differentiation, which is the reprogramming of an adult cell to directly become another adult cell type without reversing back to an intermediate Pluripotent state (Trans-differentiation IP). This early work was late 1990s to early 2000s...
As mentioned the term Pluripotency is by definition the ability to derive stem cells of any type. The Technology IP in the sector is how to create Pluripotent cells and then also how to maintain and differentiate those Pluripotent cells into the desired cell types and then scale those sufficient for Therapeutic marketing at a reasonable cost... As an analogy think of this as a Tree with a few Roots only that feed a Trunk - the Trunk is the Pluripotent state from which there emerges a number of Branches, which represent the Multipotent states, which then sprout Sub-Branches and then the leaf bearing Offshoots of those which produce the Leaves.... These Leaves are the finished individual cell types.. This is where the analogy breaks down somewhat as the Main Branches in the Tree, the Sub-Branches, the Offshoots and the Leaves would all be different colors representing different states along the unique path to the final cell types, nor does it represent the accurate number of possible intermediate step stages to get to the end result Cell Type - anyway you get the point I hope..
Human Embryonic Pluripotent Cells (hESCs) can be made by anyone soon given Thompson's WARF hESC derivation process is coming out of Patent Protection. That will open up hESC science to more innovation and programs - but it is a destructive process. In Europe the WARF Patents were cancelled and therefore the hESC research field was pursued with more interest than it was in the US. As everyone knows OCATA invented a unique approach to derive an hESC by way of a non-destructive process - Blastomere IP. That is what sets OCATA apart in getting to the Pluripotent Trunk stage using the embryo Root. No one else does that and Ocata have a Patent in the US and Patent Applications submitted in other Territories to protect that hESC advantage. In Europe a non-destructive technology is required to pursue Patentability - OCATA awaits the European Legal process on its Blastomere Patent Application to secure its rights there. This is apart from the differentiation IP protocols of taking a Pluripotent cell and making it differentiate into a number of different Multipotent cell lineages and end cell types. These are what ACTC has a unique Priority based ability to develop and is pursuing broad Patent IP on for the Eye, Blood and Immune System areas... This positioning is unique in the world of hESC science for those areas of development IMO.
In respect to iPS Ocata is looking to secure the "Pluripotent" definition inclusion in their more recent Patent Extension Applications in all the above program areas as a right from the Original Filings and the inclusive Legal interpretation of the term "embryonic" in recent rulings. This has already been established in Australia for the Eye Program. The term iPS is a term coined by Yamanaka and as I relayed refers to taking an Adult Cell and converting it with reprogramming steps to a Pluripotent state - this is another of the Roots of the Tree Trunk. OCATA and others were doing this long before Yamanaka - OCATA's term for that was "De-differentiation" and the components of that technology are Patent Filings with v.early Priority Dates (late 1990s and early 2000s). A number of Patent Filings relate and ref to OCT4 as a differentiation / reprogramming protein used in the technological processes and methods that ACTC and others were experimenting with. In 2006 Yamanaka defined a set of 4 factors (incl. OCT4) that if used according to his protocol would convert Adult Cells back to a Pluripotent state. The fact that he documented and published this protocol in the Literature and made it widely available as a process for Lab Researchers made it a "standard" and won him the Nobel (rightly so). However, it doesn't negate the fact that other scientists had been working on the same reprogramming language, some way before Yamanaka (incl. OCATA). This is what Mgt. have been saying and that it has an early Priority on the use of OCT4 in the science. This of course relates to those using OCT4 in their protocols for iPS - however, it is an evolving field so the use of OCT4 in certain Protocols isn't necessary... I've stated my opinion on OCT4 in this iCell Thread.
Gary Rabin, then CEO of OCATA, relayed in an interview that OCATA's iPS approach was "zero-footprint reprogramming using vectorless technology" and is more to the point here as it's a reference to the difference in approaches being played out in the iPS field currently... Rather than getting caught up in defending the OCT4 Priority - which IMO Big Pharma partners will do later, if necessary - the current issue is how well do the various protocol approaches perform... That is still an open question but certainly I think most would agree now that an integration free route (i.e. not entering the genome) is safer - hence the effort to use steps that avoid integrating. However, the use of vectors (irrespective of Viral use or not) is also at issue here and it's been shown in certain cases to create residual effects. Yamanaka and Thompson use vectors. The leading safety protocols are the Harvard related iPS teams - Daley's mRNA process and Kim/OCATA's protein method... Safety first is the goal - hence the keen interest in Research Licenses for freely available integrating and non-integrating vector based iPS lines (Yamanaka and Thompson) but slow progress on Therapeutic Programs based on same... It is still too early to determine which Pluripotent route is best suited for mass market therapeutics but clearly OCATA is amongst the handful of leaders in the field and one could say is the frontrunner with it's safety first iPS protocol pending IND submission selecting an enucleated cell type (Platelets) as a further safety precaution. In addition, OCATA has a potential lock on the Pluripotent RPE derivation that the other front runner, Yamanaka, is pursuing as his first indication, which would add distance to OCATA's first mover position...
In summary OCATA's Pluripotent strategy is to link this fundamental Trunk source for cell derivations lineages for the Eye, Blood and MSC Programs. Apart from that the Pluripotent protocols are valuable as stand alone Root Platforms - Blastomere, iPS Protein Reprogramming, SCNT and Parthenogenesis. These foundational Root technology approaches, plus Trans-Differentiation tech, make up the knowledge base and IP of the company generally and are interwoven as part of its Scientific Tool Kit. The breadth of the science IP protects the company from sector challenges - even if it's not using a part of the technology IP vault for a specific program.... Patents granted are more valuable than Applications of course but in this field knowledge and innovation are the keys to success given it's moving so quickly - a first mover advantage with IP is a powerful force.
To conclude it has been said that "Ocata have some of the best developmental biologists in the world" and it's clear they have IP in all Pluripotent technological avenues and are pioneering the science with a first mover strategy in these Root processes, which therefore suggests that if any one process is successfully translated this will establish OCATA's future and those associated.
Cheers
Root Pluripotent Processes:
This video below of Dr. Lanza's scientific presentation goes into detail about the various steps mentioned in the summary above - note that the Doctor talks about all Root IP approaches as part of his scientific knowledge base toolkit (hESC, SCNT, iPS and by default Parthenogenesis when referring to the Egg - see below Patent App).
The Cytoplasm is nature's incubator. What Ocata have here is a foundational approach to reprogram an old adult cell backwards in time to be youthful via a young new Egg, along the way using technology to modify.. This effectively is the road to healthy longevity via a natural cell regression mechanism that uses you're own adult cells in further steps to generate pluripotent cells for therapeutics... Previously OCATA needed access to a constant supply of human eggs and therefore it wasn't viable - now it is. SCNT is the term used for this as it stands for Somatic Cell Nuclear Transfer and is now to be combined with OCATA's iPS and Parthenogenesis Platforms using ES culturing differentiation technology for patient specific cells and tissues as an alternative route to deliver Cell Therapeutics. Genetic modifications are part of the overall process generally...
Effectively this states that ACTC uses Eggs to nurture regression, then uses SCNT technology or Parthenogenesis to derive "embryonic" cells for Therapeutic use... Before the lack of human Eggs was the bottleneck and the ethical issues associated with creating human embryos. With this technology approach Ocata have choice and control over the entire process of developing Pluripotent cells without human fertilized embryos and without the possibility of viable embryo by products... iPS allows the reversion an Adult cell to become an Egg and with controlled stimulation "embryonic/Pluripotent" cells can be produced without any possibility of developing viable human embryos... This is Lanza's scientific stem cell tool kit brilliance and what makes OCATA unique in that it has ALL the necessary pieces of the puzzles and knows how to assemble them.
Further General References:
Embryonic stem cell - Wikipedia, the free encyclopedia
Induced pluripotent stem cell - Wikipedia, the free encyclopedia
Somatic-cell nuclear transfer - Wikipedia, the free encyclopedia
Parthenogenesis - Wikipedia, the free encyclopedia
Transdifferentiation - Wikipedia, the free encyclopedia
Adult stem cell - Wikipedia, the free encyclopedia (VSELs)
Added Dr. Zarbin's papers & presentation:
http://www.revophth.com/content/d/retina/c/37809/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176062/
http://www.asorn.org/client_data/fil...zarbin2012.pdf