The Coming of Age of Pluripotent Science & Musings on a Sonogram
One of the most memorable images I can recall on the effect of catalytic dynamics for me during these years of scientific curiosity was the explosive result of sperm enzyme successfully impacting a human egg - almost Big Bang like in all its microscopic potential.
 Signals of Canada, a leading destination for "insiders’ perspectives on the world of regenerative medicine and stem cell research, written by scientists and professionals in the field" is hosting a "Blog Carnival" of which this article is one of a number being written covering the iPSC anniversary topic. Please click here to read what other bloggers think.
Signals of Canada, a leading destination for "insiders’ perspectives on the world of regenerative medicine and stem cell research, written by scientists and professionals in the field" is hosting a "Blog Carnival" of which this article is one of a number being written covering the iPSC anniversary topic. Please click here to read what other bloggers think.
 Inherently complex the various Pluripotent states and the multitude of their progressively differentiated descendants, as they relate to human biological microsystems, have only begun to be explored and understood. The inherent processes by which these interconnected derivative cells work and communicate are by and large just now being decoded and mapped. Although it’s been only ten years since the discovery of iPSCs and nearly 20 years since the human embryonic stem cells were first isolated the progress made to-date in translating Pluripotent science into real world clinical programs is very much the focus now of countless labs in the field, thanks in large part to the advent of “open source” iPSC technology.
Inherently complex the various Pluripotent states and the multitude of their progressively differentiated descendants, as they relate to human biological microsystems, have only begun to be explored and understood. The inherent processes by which these interconnected derivative cells work and communicate are by and large just now being decoded and mapped. Although it’s been only ten years since the discovery of iPSCs and nearly 20 years since the human embryonic stem cells were first isolated the progress made to-date in translating Pluripotent science into real world clinical programs is very much the focus now of countless labs in the field, thanks in large part to the advent of “open source” iPSC technology.
Prior to iPSCs the use of hESCs and the technology associated with its clinical translation was largely a specialty area limited in scope by funding, rules, regulations and IP. The advent of iPSCs changed that and with it the stem cell industry added a universal layer of potential. Whether it be using Pluripotent derived cells as tools or more notably to develop therapeutic cell candidates for clinical use those researching and developing applications using these cells are pioneering the way forward for the emerging era of next generation stem cell products.
To put not too fine a point on it, we have only scratched the surface and in the next ten years I expect there will be a number of Pluripotent treatments on the market in various countries and many many more still in the clinic moving towards approval with positive results over standard of care or filling-in where there is currently nothing to offer patients in need.
When I recently scanned the Pluripotent sonogram I saw some Art where 4 heads appeared! Unlike our shock horror double take when 2 appeared in my wife’s scan, I was glad to see the 4 represented there, happily squished together and well. One seems to be growing bigger at the expense of a couple of the others but by and large it’s a Bridge Gang willing and able to take on the challenges when alive and kickin. I personally look forward to seeing them all born healthy and grow, in addition to their Olympic caliber Adult cousins. That would give us semi-oldies the best chance when it´s our turn to ask for help from those wise and experienced in the Jedi ways of healing. This is where Advocacy for Cures comes in.
Hope is a powerful force and will always be there for patients in need. Unfortunately the reality is that most next generation Pluripotent cell solutions are still a few steps away, if not more, for those that suffer. High science, low science, no science - too many people continue to be excluded, lack alternatives, suffer & die from disease. Stem Cell Science offers potential solutions and requires stakeholders to rally around programs and data that deliver real world results, even marginal benefits over existing options while awaiting more advanced solutions.
International Team Derives Haploid Pluripotent Stem Cell Lines with Full Plasticity
 |
| usnews |
One can now extend that impact phenomena analogy to the very pertinent research and translational effect Induced Pluripotent Stem Cell (iPSC) technology has had on the field of molecular biology and regenerative medicine.
 |
| Nature - Andy Potts |
It’s been ten years since Shinya Yamanaka and Kazutoshi Takahashi opened the portal to a whole new way of thinking and practicing the Art of Stem Cell Science by announcing that embryonic like properties could be regained in adult cells through molecular reprogramming.
 |
| Royal Society |
Much has been written about this apex moment of foundational innovation which ushered in the era of mainstream adoption of reverse engineering techniques on human cells but as we celebrate the ten year anniversary of iPSCs the opportunity presents itself to reflect and celebrate the coming age of Pluripotent science, specifically iPSCs.
 Signals of Canada, a leading destination for "insiders’ perspectives on the world of regenerative medicine and stem cell research, written by scientists and professionals in the field" is hosting a "Blog Carnival" of which this article is one of a number being written covering the iPSC anniversary topic. Please click here to read what other bloggers think.
Signals of Canada, a leading destination for "insiders’ perspectives on the world of regenerative medicine and stem cell research, written by scientists and professionals in the field" is hosting a "Blog Carnival" of which this article is one of a number being written covering the iPSC anniversary topic. Please click here to read what other bloggers think. Inherently complex the various Pluripotent states and the multitude of their progressively differentiated descendants, as they relate to human biological microsystems, have only begun to be explored and understood. The inherent processes by which these interconnected derivative cells work and communicate are by and large just now being decoded and mapped. Although it’s been only ten years since the discovery of iPSCs and nearly 20 years since the human embryonic stem cells were first isolated the progress made to-date in translating Pluripotent science into real world clinical programs is very much the focus now of countless labs in the field, thanks in large part to the advent of “open source” iPSC technology.
Inherently complex the various Pluripotent states and the multitude of their progressively differentiated descendants, as they relate to human biological microsystems, have only begun to be explored and understood. The inherent processes by which these interconnected derivative cells work and communicate are by and large just now being decoded and mapped. Although it’s been only ten years since the discovery of iPSCs and nearly 20 years since the human embryonic stem cells were first isolated the progress made to-date in translating Pluripotent science into real world clinical programs is very much the focus now of countless labs in the field, thanks in large part to the advent of “open source” iPSC technology. Prior to iPSCs the use of hESCs and the technology associated with its clinical translation was largely a specialty area limited in scope by funding, rules, regulations and IP. The advent of iPSCs changed that and with it the stem cell industry added a universal layer of potential. Whether it be using Pluripotent derived cells as tools or more notably to develop therapeutic cell candidates for clinical use those researching and developing applications using these cells are pioneering the way forward for the emerging era of next generation stem cell products.
To put not too fine a point on it, we have only scratched the surface and in the next ten years I expect there will be a number of Pluripotent treatments on the market in various countries and many many more still in the clinic moving towards approval with positive results over standard of care or filling-in where there is currently nothing to offer patients in need.
The topic of what can we expect to see on the frontline of the developing therapeutic market using Pluripotent derived cell products is often highlighted as a discussion point and rightly so given the limited public depth of awareness on the subject and the long standing promise by the sector as a potential basis for effective treatments.
Below are some of the targets and iPSC programs representative of the state of play in the field to look out for:
| TARGET - COMPANY/INSTITUTION - LEAD SCIENTIST(S) - CELL - DISEASE - AUTO/ALLO* | ||||||
| EYE | ||||||
| Riken/Healios | Masayo Takahashi | iRPE++ | Wet/Dry AMD++ | Auto>Allo | ||
| Notes: Program will address many disease states of Retina/Eye | ||||||
| Astellas RegMed | Lanza/MacLaren | iRNP++ | Dry AMD/RP++ | Allo | ||
| Notes: Program will address many disease states of Retina/Eye | ||||||
| UWisconsin Waisman | Gamm/Meyer | iRNP/RPE | Stargardt+ | Allo | ||
| Notes: Representative of next gen concept using eye "organoids" | ||||||
| NEI/CDI-Fuji | Bharti/Miller | iRPE | Dry AMD | Auto | ||
| Notes: US Govt backed program | ||||||
| Cedars Sinai/CIRM | Shaomei Wang | iRNP | RP | Allo | ||
| Notes: Advanced status w/ IND enabling studies | ||||||
| BRAIN | ||||||
| Kyoto Univ | Jun Takahashi | iNC | Pakinsons | Allo | ||
| Notes: Leading iPSC Parkinson program due to start in 2017 | ||||||
| Sloan Kettering | Lorenz Studer | eNC/iNC | Pakinsons | Allo | ||
| Notes: Top tier US hESC/iPSC lab moving to clinic | ||||||
| Scripps/CIRM | Jeanne Loring | iNC | Pakinsons | Auto | ||
| Notes: Bringing it home full circle w/ CIRM onboard | ||||||
| IMMUNE | ||||||
| Cynata | Slukvin/U Wisconsin | iMSC | GvHD++ | Allo | ||
| Notes: Entering clinic later in 2016 w/ solid pre-clinical data | ||||||
| Astellas RegMed | Robert Lanza | iMSC | Sepsis, Lupus++ | Allo | ||
| Notes: Multiple targets across board w/ pre-clinical hPSC data | ||||||
| BLOOD | ||||||
| Megakaryon-Kyoto/Tokyo+ | Eto/Nakauchi/Daley | iPlatelets | Cancer/Surgery++ | Allo | ||
| Notes: Leading Japanese program poised to enter clinic in 2017 | ||||||
| Novosang-Roslin/SNBTS++ | Marc Turner | iRBC | Thalassaemia++ | Allo | ||
| Notes: Leading UK Consortia looking to clinic in 2017 | ||||||
| Inserm/PlatOD | Dominique Baruch | iPlatelets | Cancer/Surgery++ | Allo | ||
| Notes: Leading French program nearing clinic in 2017 | ||||||
| Astellas RegMed | Robert Lanza | iPlatelets | Cancer/Surgery++ | Allo | ||
| Notes: Had a leading program using research grade iPSC line in 2013 | ||||||
| Players in CAR/Immuno Space | iBloodCells | Cancer/Immune | Auto>Allo | |||
| Notes: Auto/Allo B, T, NK, DC+ benefits 4 immuno product requirements | ||||||
*The above listing is representative of the sector and is not at all comprehensive. Apologies to the many great programs that should be there also.
hiPSC science has industry wide support globally and is a mainstream technology acceptable in jurisdictions in which other ES methods face challenges. Translational hurdles for hiPSC are specific to their reprogramming and to the adult to youthful conversion which forms the basis of the applied technology. This presents an additional safety component to the already strict regulatory oversight applied to the clinical translation of Pluripotent programs now and in the future.
hiPSC science has industry wide support globally and is a mainstream technology acceptable in jurisdictions in which other ES methods face challenges. Translational hurdles for hiPSC are specific to their reprogramming and to the adult to youthful conversion which forms the basis of the applied technology. This presents an additional safety component to the already strict regulatory oversight applied to the clinical translation of Pluripotent programs now and in the future.
Will they be highly successful and achieve revolutionary paradigm shifting status and establish new standards of care in their go-to-market quests?
That is a subjective question for each and every program and one which you could speculate on, yet it would be Hype to suggest definitively without established patient data. However, the indicative MOA and technology basis of those programs on the list point to a sound foundation to work from.
That is a subjective question for each and every program and one which you could speculate on, yet it would be Hype to suggest definitively without established patient data. However, the indicative MOA and technology basis of those programs on the list point to a sound foundation to work from.
In my opinion, Pluripotent science, specifically hiPSCs as a universal technology, has the very best chance to score across the board wins for the patient in areas of unmet medical needs.
Why?
Some of the reasons I have doggedly believed that lie in the very nature of the plasticity of the sources, youthful phenotypes, cell expression and innate modulatory properties. Other reasons specifically relate to the field’s capacity to precisely derive and modify them in-vitro while perfecting their required derivative purities and expandability to consistently replicate them indefinitely in volume under strict quality control for regulated mass market applications.
This potential, if successfully delivered together, will usher in a new Pluripotent Era in the Stem Cell Story.
Why?
Some of the reasons I have doggedly believed that lie in the very nature of the plasticity of the sources, youthful phenotypes, cell expression and innate modulatory properties. Other reasons specifically relate to the field’s capacity to precisely derive and modify them in-vitro while perfecting their required derivative purities and expandability to consistently replicate them indefinitely in volume under strict quality control for regulated mass market applications.
This potential, if successfully delivered together, will usher in a new Pluripotent Era in the Stem Cell Story.
Commentary
Are we at an inflection point?
Are we at an inflection point?
Certainly the perceived slow pace of translational activities has been a media drag on the sector, irrespective of the actual comparative timelines to move from bench to bedside. In retrospect there are still only a surprisingly small number of clinical programs in trials worldwide using Pluripotent derived cells, due in large part to stricter preclinical and regulatory standards applied to safety issues associated with these cell sources. So one would say we’re definitely due some momentum building inflection points….
Human embryonic stem cell trials were initiated in the US some 6 years ago on the basis of only research cell lines after lengthy preclinical research and safety checks. Subsequent small studies in Europe and Korea using hESCs added additional safety data. These trials paved the way for iPSCs yet still today the only enrolled clinical trial for iPSC derived therapeutics is in Japan. This pioneering trial had been on hold for over a year as the cell source analysis flagged possible genetic instability issues and was switched out from an autologous approach to a recently approved allogeneic cell line and is due to resume in 2017. The one patient to-date receiving the iPSC based iRPE cell sheet for Wet AMD has been reported to be in good condition with no apparent safety issues associated with her procedure.
The rigorous standards of the need to use an NIH approved clinical grade cell line in the US has delayed the start of US trials on iPSCs and only just recently has one been announced as available. Pre-clinical lab work one would assume would now need to be done using that line for the clinical trial programs wishing to enter the clinic sometime in the future (yrs) or approval sought and granted for proprietary lines already used for clinical prep on existing developmental programs. This safety issue, albeit necessary and prudent, has forestalled the advancement of Western work and raised the bar from where hESCs entered the clinic.
When discussing timelines and where this segment of the sector is headed it’s important to factor in these types of regulatory hurdles one must overcome on the road to a Pluripotent IND, clinical trial approval and human phased testing. Trial design considerations require stringent oversight monitoring of Pluripotent trials and have and will slow down the translational best efforts of those academic and commercial players entering the space.
Generally if it takes longer that the average drug development process to see stem cell based products enter trials and progress through the Phases and a therapeutic emerge from any stem cell specific regulatory approval pathway people will continue to be disappointed. A scaled approach to the sector’s product entry and exit criteria seems reasonable given the variance of risks associated with different stem cell products under some form of adaptive umbrella.
Safety is of the essence and the nascent SC sector requires everyone err on the side of caution. This is the mantra I hear regularly and I can’t really say it’s not appropriate to a great extent. However, it is this writer’s opinion that the priority should not be overly weighted towards the slowest approach to protect the sector at the expense of patients willing to engage in regulated, open and comprehensive phase development programs designed to enroll and prove the science. Sufficient data can only be generated from a participatory system that is adaptive and accommodating not restrictive and burdensome by design or intent.
A Quad Pregnancy demands care & attention.
A Quad Pregnancy demands care & attention.
 |
| Ashley Gardner Quad IVF Reaction cnn |
 |
| buzzfeed |
To conclude I have reported on the stem cell industry, specifically the Pluripotent segment for many years, and it has been easily influenced by sentiment and competitive currents rather than sharpening its aim on achieving sector growth. What we all care about most are real solutions for the long list of conditions that continue to ail us and for those that we love. Rather than future technology leading, current programs built on the foundation of pioneering efforts in both the Adult and Pluripotent fields need to be clinically accessible and then successfully delivered widely. Supporting and driving all safe and effective stem cell solutions will propel the entire sector forward.
Safety with pace, open and inclusive. Driving patient centric solutions forward as a community, in a modernized regulatory environment, by design and for the people.
Advocacy for Cures.
Cheers
Refs:
Cell Press Nucleus - "iPSCs: A Decade of Discovery" (comprehensive review issue)
Cell Stem Cell Editorial: "10 Questions: Clinical Outlook for iPSCs" Cell Stem Cell, Vol 18, Issue 2, 170-173, DOI: 10.1016/j.stem.2016.01.023 (included in review issue ref above)
Ilic, D. and Ogilvie, C. (2016), "Human Embryonic Stem Cells — What Have We Done? What Are We Doing? Where Are We Going?". Stem Cells. doi:10.1002/stem.2450
The Niche, P.Knoepfler: "Yamanaka's baby turns 10 so here's a top 10 list of IPS cell hot button bullet points"
Refs:
Cell Press Nucleus - "iPSCs: A Decade of Discovery" (comprehensive review issue)
Cell Stem Cell Editorial: "10 Questions: Clinical Outlook for iPSCs" Cell Stem Cell, Vol 18, Issue 2, 170-173, DOI: 10.1016/j.stem.2016.01.023 (included in review issue ref above)
Ilic, D. and Ogilvie, C. (2016), "Human Embryonic Stem Cells — What Have We Done? What Are We Doing? Where Are We Going?". Stem Cells. doi:10.1002/stem.2450
The Niche, P.Knoepfler: "Yamanaka's baby turns 10 so here's a top 10 list of IPS cell hot button bullet points"
____________________________________________________________
____________________________________________________________
International Team Derives Haploid Pluripotent Stem Cell Lines with Full Plasticity
Pluripotent stem cells have been an invaluable tool to progress fundamental basic research. There isolation and maintenance in animal and human cell lines have furthered the study of cell systems and their function, fueling the rapid rise of scientific discovery and translational application.
Recent news points to a significant addition to the pluripotent landscape. Scientists at the Hebrew University of Jerusalem, Columbia University Medical Center (CUMC) and the New York Stem Cell Foundation Research Institute (NYSCF) have presented a novel method to add to the existing pluripotent toolkit by creating a new form of human embryonic stem cell with the capacity to develop all three germ layers in-vitro and in-vivo. The teams’ findings were reported in the journal Nature¹.
Uniquely, the work presented by the researchers sets a benchmark precedent given these new types of ES cells are genomically haploid and surprisingly are able to divide and renew as per existing pluripotent cells for over 30 passages, while retaining cells of a normal haploid karyotype. The researchers achieved this by using an established chemical method of parthenogenetic stimulation of human oocytes to activate them into false fertilization. The resulting cell cycles were sorted at regular intervals to screen for diploidization, which occurred at a rate of 3-9% cells per day. Use of in-situ FISH florescence and centromere protein foci analysis enhanced accuracy of the sorting process. The new cell lines created exhibited similar to diploid transcriptional and epigenetic definitions of pluripotency. They also tested for self-renewal and full lineage specific differentiation capability (neuronal, cardio and pancreatic data shown plus teratoma assay) while maintaining a haploid specification.
Analysis of the differential of same cycle cells between haploid and diploid gene profiles using transcriptome RNA sequencing correlated to a distinct difference in up regulation and down regulation patterns. This divergence equated to a twofold change in haploid to diploid genetic signatures, with distinct X chromosomal over weighting and expression in haploid up regulated genes, representative of diploid X chromosomal inactivation in one of the two X chromosomes. Noted via autosomes transcriptome and methylome analysis the researchers pointed to the up regulation in haploid cells of 11 genes involved in oxidative phosphorylation plus all 13 mitochondrially encoded oxidative phosphorylation genes, indicative of nuclear and mitochondrial coordination. This was represented in a reported 32% increase in mtDNA to nuclear DNA ratio. Physically, haploid cells were comparatively smaller in size and in volume ratios.
Previous mammalian cell lines of this type have been generated but to-date only diploid ES lines have been realized from human germ cells. One such human cell line was used to derive neural stem cells (hpNSCs) and has entered the clinic this month in a Phase 1 study for Parkinson’s disease.
The team highlighted the utility value of the pluripotent haploid cells, stating: “haploid mammalian cells have proven invaluable for loss-of-function screens” and the “discovery of haploid human ES cells should thus provide novel means to delineate basic aspects of human genetics and development.”
As a community the advent of ubiquitous gene editing technology and increasingly varied human cellular development tools, such as this method, opens up exciting new opportunities for biological discovery in human model systems.
Cheers
Reference – (1) L. Sagi et. al. Derivation and differentiation of haploid human embryonic stem cells. Nature, 2016; doi: 10.1038/nature17408.
_________________________________________________________________
Naїve Human Pluripotency & The Broad Shoulders of Science
 |
| Stevenage, UK BioScience Campus |
Scientific debate in the pursuit of knowledge by way of accumulated evidential data is fundamental, just as socio-economic competition is needed to spur innovation, product development & growth in commercial business. Distinct and largely operating on their own, these two worlds have now collided and become integrated in a synthetic process that is driving 21st century evolution.
As a pillar of progress and community success, medical science is a central focus of tomorrow’s design. One in which the health and well-being of society can be calculated and factored into the spreadsheets of sustainability. The footnotes in such macros are bolded as requirements to achieve, yet are a challenge to deliver.
 |
| Salk iPS_Ruiz-StemCell |
Source, Stability and Scale – the trinity of our destiny caught in a matrix of possibilities where clarity of method is needed.
Science knows no bounds when it comes to unresolved issues of definition and process, so the discussion continues. However, with advents in genomic analysis the cell systems of our inner being are becoming clearer and these new insights are helping to provide the answers.
The human "naїve" cell state in the earliest stages of human embryogenesis is one such focus. The identification and establishment of cell lines along the pluripotent continuum has been a foundational endeavor of the community. Ever since the mouse modelling proved the existence of these powerful engines of growth have the leading labs sought to isolate and engineer the human equivalents. This ongoing work has inspired the field to challenge each other to discover and answer the unresolved questions that will unlock the full potential of pluripotency.
 |
| Whitehead Institute MIT |
Over the years I have looked for data on early stage embryonic states, specifically any variations in the genetic profiles of pre-compaction blastomeres and ICM hESCs. The Galan, Á. et al (2010) Valencia paper was one such document I found. Of note here in the more recent research done on early human development was that the variation in profiling was correlated to naїve at a specific stage of human embryogenesis at around the 8 cell stage (referred to in Q&A). This moment evidently coincides to the withdrawal of maternal influence yet prior to the blastocyst wave of fate expression.
 |
| Benjamin Dodsworth |
 |
| Sally Cowley Ph.D |
I connected with Ben in a Twitter exchange and he was open to doing a Q&A on the topic, which we started prior to some subsequent developments in the area (iPS “2C” totipotent reprogramming² and the Karolinska paper³ on early human development). Comments on the 2C paper are included in the interview below in [brackets].
Thank you Ben for your feedback & good luck with your research.
Cheers
Q&A:
M - With regard to the human Naive state generally and attempts made to create hNaïve cell lines, are we really mainly discussing iPS reprogramming techniques to revert to an earlier point of embryogenesis or would you envision a new methodology for ICM hESC cell lines with them being converted backwards post extraction also? If so do you envision any technical issues associated with than or in their maintenance?
B - Very good point. There are clear parallels to iPS reprogramming techniques. We are currently looking at a method to convert already established human pluripotent stem cell (hPSC) lines to the naïve state. However, if the naïve state is indeed as useful as we anticipate and becomes our new standard, I would expect the emergence of protocols to generate naïve induced pluripotent stem cells directly from primary cells (such as fibroblasts) which skip the primed state. If this holds true, I do expect technical issues. Many protocols for handling hPSCs have been optimised for cells in the primed state. These will not be ideal for naïve cells. Maintenance of naïve human cells might also be challenging and current standard operating procedures will have to be adapted.
M - You mention hESC differentiation pathways that are unreachable - which are those?
B - Endodermal and germline lineages are difficult to access with our current primed hPSCs. This means that although possible, it is inefficient. The Hanna lab have actually used naïve cells to generate primordial germ cells (PGCs) very efficiently. In comparison, primed cells do not efficiently differentiate into PGCs.
Just as important as accessing these differentiation pathways is the maturity of the cells we then produce. Maturity is the extent to which their functions resemble the in vivo cell type. Naïve hPSCs might increase the level of achievable maturity (for example of hepatocytes).
But what I find a lot more interesting is that we have excellent protocols for the differentiation into cells (for example dopaminergic neurons) which work robustly with some hPSC lines but not with others. This heterogeneity could be removed with a protocol which uses cells that are developmentally at the same starting point and without epigenetic bias. The naïve state could deliver on both of these aspects.
M - Has there been any focus on comparative analysis done using hESCs derived from various cell stages of the early human embryonic Blastomere cell stages 2, 4, 8, 16?
B - To my knowledge this has not been performed using hESCs derived from different developmental time points. However, a very useful direct comparison of current naïve and primed hES lines to early human embryonic blastomere cell stages has been performed using single cell transcriptomics by Huang, Maruyama, and Fan⁴ (go directly to Figure 2B). They used datasets from Vassena et al.⁵, 2011, Xie et al.⁶, 2010 and Yan et al.⁷, 2013 and compared gene expression to various naïve cells.
M - Why is the Naive state also referred to as Ground State? Is there any technical reason?
B - Ground state and naïve state both describe the earliest accessible and unbiased cellular state. These terms are interchangeable.
M - Do you believe the reprogramming concept being studied will ultimately be pursued to the point where reversion produces a Totipotent state in order to fully map the process?
B - Possibly, but there are many technical hurdles to overcome and ethical issues to consider.
[M - Do you have a as follow-up comment on this point with regard to the recent Inserm Totipotent development?
B - The 2C paper is indeed a very interesting piece of work which I have been following closely. However, I would like to see more evidence for totipotency, in particular higher efficiency differentiation down difficult lineages such as PGCs. There is not enough evidence to show that these cells are indeed totipotent. For our lab, totipotent cells are unnecessary and we won’t be using these.]
M - Do existing techniques adequately result in Pluripotent cells able to be scaled and applied effectively to therapeutic programs?
[M - Do you have a as follow-up comment on this point with regard to the recent Inserm Totipotent development?
B - The 2C paper is indeed a very interesting piece of work which I have been following closely. However, I would like to see more evidence for totipotency, in particular higher efficiency differentiation down difficult lineages such as PGCs. There is not enough evidence to show that these cells are indeed totipotent. For our lab, totipotent cells are unnecessary and we won’t be using these.]
M - Do existing techniques adequately result in Pluripotent cells able to be scaled and applied effectively to therapeutic programs?
B - Current techniques allow the production of induced pluripotent cells to be scaled up. However, before iPS cells can be used therapeutically, the field needs to overcome some fundamental issues. Two main challenges revolve around the host eliciting an immune response to hES or iPS cells even when sourced from the same individual and on the other hand, pluripotent cells have been changed to allow proliferation. This raises concern that these cells could be more susceptible to becoming cancerous. There is a lot of preclinical work to be done.
M - In your conclusion you point to the protocols yielding different results which has yet to be interpreted conclusively, as well as the transient nature of the actual biological moment in-vivo which it may occur. In addition you point to the possibility of a scale of different states along the defined continuum. In that respect would you say any in-vitro activity to reproduce these embryo-genesis states are by defacto man made events and the best we can expect ultimately is a "like" status?
B - Absolutely. Any cell grown into the lab is unlikely to be exactly the same as the in vivo counterpart. As long as we keep this in mind and factor it into our data interpretation, this is not a problem.
M - The data you cite regarding the primate transcript HERVH indicates that mouse systems are distinct to that of primates in this specific area (at least that monkey species). This would indicate that aspects of the human embryo-genesis system are biologically different to that of mouse, in certain ways. Does that perhaps also apply to cell prodigy behavior in your opinion?
B - The paper discussing HERVH is an excellent piece of work which shows compellingly that pluripotency networks are indeed different between human and mouse. And you are right, we can also see these differences in the cellular behaviour. Mouse and human ES cells cannot be cultured in vitro in the same way. The networks which allow capture of naive pluripotency in mouse are not identical to the human system.
M - The utility advantages you mention of Naive versus Primed indicate manufacturing bias towards use of Naive in the future. Can you outline the utility issues specifically for naive cell use and do you view this for specific clinical purposes or for certain discovery processes.
B - Although some labs are currently working on clinical applications, we are focusing on using hPSCs for modelling only. The human naive state promises a lot of benefits – if it is indeed similar to the naive state in mouse. Extrapolating from the mouse, homogeneity would be expected to be improved in naive cell populations. This means that cells are held not in a spectrum of states but all at exactly the same developmental time point. Differentiation protocols could be a lot more effective when applied to a uniform starting point. Other benefits include higher cell yields due to faster doubling times and easier handling.
M - The statement that "TGFβ might not be essential in the human system" caught my attention. Can you elaborate on that in light of published data.
B - In the past, TGFβ signalling was required to maintain hPSCs in culture. However, the requirement of TGFβ signalling is a trait associated with the primed state. In addition, the inhibition of TGFβ signalling increases efficiency of mouse iPSC reprogramming. This is why it would be interesting if we can culture hPSCs without TGFβ.
##
[Follow-up Q relating to the Karolinska analysis paper³ on early human development was left unanswered prior to publishing]
Q&A Refs:
1. Dodsworth, B. et al. (2015). The Current State of Naïve Human Pluripotency. Stem Cells. doi: 10.1002/stem.2085
2. Ishiuchi, T. et al (2015). Early embryonic-like cells are induced by downregulating replication-dependent chromatin assembly. Nature Structural & Molecular Biology 22, 662–671 (2015) doi:10.1038/nsmb.3066
3. Töhönen, V. et al. Novel PRD-like homeodomain transcription factors and retrotransposon elements in early human development. Nat. Commun. 6:8207 doi: 10.1038/ncomms9207 (2015).
4. Huang, K. et al. (2014). TheNa ve State
5. Vassena, R. et al. (2011). Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development Development 138, 3699–3709
4. Huang, K. et al. (2014). The
5. Vassena, R. et al. (2011). Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development Development 138, 3699–3709
6. Xie, D. et al. (2010). Rewirable gene regulatory networks in the preimplantation embryonic development of three mammalian species" Genome Res. 20, 804–815.
7. Yan, L. et al. (2013). Single-cell RNA-Seq profiling of human pre-implantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 20, 1131–1139.
Selected Other Refs (in no particular order):
Selected Other Refs (in no particular order):
Takahashi/Yamanaka review of the iPS reprogramming pluripotency
Takahashi,K., et al. A developmental framework for induced pluripotency. Development 2015 142: 3274-3285; doi: 10.1242/dev.114249
______
Salk paper on region specific PSCs (2015):
Wu, J. et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature 521, 316–321 (21 May 2015) doi:10.1038/nature14413
_______
Genomic analysis using single cell RNA (2013)
Xue, Z. et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 500, 593–597 (29 August 2013) doi:10.1038/nature12364
_______
Naive cells in hESC culture using a HERVH promoter & gene analysis of ICM & early embryo cells
Wang, J. et al. (2014). Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature 516, 405–409, doi:10.1038/nature13804
_______
1st Naive Paper MIT (w/ Hanna now in Israel, Weizmann)
Hanna, J. et al. (2010). Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A. 2010 May 18; 107(20): 9222–9227.
_______
A.Smith Cambridge downstream transcription factor Tfcp2l1 in Naive conversion
Martello, G. et al (2013). Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J. 2013 Oct 2; 32(19): 2561–2574. doi: 10.1038/emboj.2013.177
______
Singapore use of 3iL creates a closer native epiblast state of pluripotency "Naive" (rewiring of regulatory circuitry)
Chan, Y-S. et al. (2013). Induction of a Human Pluripotent State with Distinct Regulatory Circuitry that Resembles Preimplantation Epiblast. Cell Stem Cell. 2013 Dec 5; Vol 13, Issue 6. doi:10.1016/j.stem.2013.11.015
______
Hanna Weizmann Institute use of 2iL & in-vitro derivation of mouse like naive cells capable of forming inter-species mouse–human chimeric embryos
Gafni, O. et al (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013 Dec 12;504(7479):282-6. doi: 10.1038/nature12745.
______
Seattle Washington alternative derivation method to Naive state
Ware, C. et al. (2014). Derivation of naïve human embryonic stem cells. Proc Natl Acad Sci U S A. 2014 Mar 25; 111(12): 4484–4489. doi:10.1073/pnas.1319738111
______
Whitehead MIT Talen mediated reporter system for naive derivation medium 5iL (R. Jaenisch)
Theunissen, T. et al. (2014). Systematic Identification of Culture Conditions for Induction and Maintenance of Naive Human Pluripotency. Cell Stem Cell doi: 10.1016/j.stem.2014.07.002
______
A. Smith Cambridge team uses simple transient expression of two transcription factors to rewire back to Naive
Takashima, Y. et al (2014). Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell, Vol.158, Issue 6, 2014 Sept 11. DOI: 10.1016/j.cell.2014.08.029
______
Valencia early embryo gene analysis
Galan, Á. et al (2010). Functional Genomics of 5- to 8-Cell Stage Human Embryos by Blastomere Single-Cell cDNA Analysis. PLOS | One 2010, Oct 26. DOI: 10.1371/journal.pone.0013615
______
Developmental biology focus on human tissue
Gerrelli, D. et al. (2015). Enabling research with human embryonic and fetal tissue resources. Development 2015, Sept 15. doi: 10.1242/dev.122820
_____
Harvard led w/ Daley/Jaenisch/Rossant - Comments by Hanna
De Los Angeles, A. et al (2015). Hallmarks of pluripotency. Nature 525, 469–478 (24 September 2015) doi:10.1038/nature15515
_____
Harvard led w/ Daley/Jaenisch/Rossant - Comments by Hanna
De Los Angeles, A. et al (2015). Hallmarks of pluripotency. Nature 525, 469–478 (24 September 2015) doi:10.1038/nature15515
________________________________________________________
World Stem Cell Policies & Regulatory Maps
In order to provide an overview perspective on the regulatory issues pertaining to the development of stem cell science I have added below two maps which show the current general legal landscape in the US and Europe.
In addition please consult with The Hixton Group for additional regulatory information in their global resource.
For Europe you can also use the following interactive map via Eurostemcell.org to read further details on individual countries and their respective legislation, which governs ES investigation within that country. This further detail may be necessary to clarify a given country's position - i.e. both Germany & Italy allow ES research but only on imported hES cell lines.
Cheers
---------------------------------------------------------------------------
Reprogramming Somatic Cells to Pluripotency
I was surprised also that the recent SCNT/iPS paper contradicted the previous research on the topic of somatic reprogramming genomic stability. What perhaps led to this surprise was the authorship of the paper, comprising 2 of the 3 lead scientists that had published significant SCNT results recently in support of SCNT technology and helped open up the possibility of its use once again as a therapeutic cell source.
The missing group of the SCNT trilogy being the S. Korean/US team - CHA & OCATA. This is apart from the representative data from the other pluripotent contender in the room hpSCs - Parthenogenetic stem cells (International Stem Cell Corp, specifically).
At this point I doubt that anyone questions any longer if SCNT is a potentially important and valuable source of ES cells, comparable to iPS. Both are "reprogrammed" from adult cells, so the similarities of origin themselves would lead one down the path to that conclusion by itself - as long as of course the correct signage is followed.
The actual reality is that SCNT was the first "reprogramming" technique to be researched before "iPS." Actually the term "dedifferentiation" was used by the early pioneers of reprogramming techniques years before Yamanaka's factors were published and he broadly heralded as the "father" of iPS. This detail is unfortunately often forgotten.
What is interesting here is that SCNT was originally presented by Mitalipov to be superior to iPS in that it's pluripotency more closely resembles IVF hESCs, while the newer paper refutes that and puts them both on an even footing in relation to each other's profiles. I think the point should be made that that SCNTs are once again being shown to be valuable here - not that they lack potential in relation to iPS. The "as good as or better than" results of the two papers confirms this - a statistical skew towards being superior actually given the two data sets presented.
The suggestion that there aren't any inherent differences between Pluripotent sources is debatable. Are we saying all humans are all basically equal if you look at us from a macro perspective and therefore when we work on a micro level, at the social and community health level, we should just remember that all are equal and no one is actually different at all? Human to human, cell to cell DNA micro analysis and epigenetics et al aside? Irrespective of patient reaction? Are we really all the same and do our cells all act the same, no matter their embryogenesis, age, expression or factors that influence them?
It seems to me that cell science is particular to the cells themselves. If an Allogeneic cell source is to treat a specific patient population it should be the best damn cell line possible, as it will be used for thousands, millions, tens of millions, even theoretically billions of people. That is what an Allogeneic cell source is made for - Universal Use (HLA matched of course). Now if you consider that cell source to be variable - which is what these studies are showing IMO - then don't you have an issue with regard to ensuring the best cell line is used? Or am I missing something here? To me it makes no difference if it's x or y line from this or that technology - the point is it needs to work and work as well as or better than the alternative.
If there is a difference in Eggs, which Mitalipov proved in his SCNT studies, and there are processing effects in the technical approaches used to create iPS cell lines, which Yamanaka himself et al. have shown, and there are genomic differences in regard to hES origin state expression, and hpSCs are HLA type and allele expressions restricted due to their parental lineage etc... then it's difficult, for me at least, to extrapolate that to "pluripotency equality" given the evidence to the contrary. I suspect this question will be debated until actual trial data is available on the head to head cell source results in comparable trial designs.
I can see there being a need to support the science broadly, which I'm all-in for, but how many scientific groups to-date have actually worked with a SCNT, Blastomere ES, Parthenogenetic or Naive iPS etc cell line in relation to and in comparison with a Blastocyst or standard OSMK/ONSL iPS research lines? Is there an equal playing field to operate?
The recent paper said SCNT is comparable to iPS and vice-versa but neither is as pristine as hpSCs. hESCs remain the gold standard, which wasn't disputed, so really nothing has changed, except hpSCs and SCNTs are being rightfully recognized, analysed and compared. More industry wide research is needed to flesh out the issues of comparative Pluripotent sources and cells lines of all lineages must be made readily available for such.
Clearly SCNT ESCs may be more difficult & complicated to create with the necessary procurement of good quality Eggs and micro surgery but they remain an equally viable alternative source - perhaps more, perhaps less than iPS, as a source for Autologous cells, given the research is still being conducted on both and both need to be tested in comparative human clinical trials along with the variations of development techniques. If both prove equally effective then iPS of course is easier to take forward to mass market industrialization. The same goes for Parthenogenetic cells. However, they will need to be clinically compared against each other and against the two+ variations of hESCs (Blastomere & Blastocyst et al). That is the only viable scientific methodology for conclusive proof of equality or superiority - safety & efficacy.
I believe there will be preferred cell sources for various reasons, assuming safety - for commercial and scalability issues. The idea that any one cell source will be discarded if it proves to be safe & efficacious isn't realistic, no matter if there are residual ethical issues or complexities of cell line creation. If there are minor differences in efficacy results across the pluripotent cell source landscape then practitioner/patient selection will be open to market forces - at it should be. Only once comparative cell source inferiority is determined will there be reason to expect a marketing withdrawal/product selection change or rejection for product authorization against an already approved stem cell therapy.
Pluripotent cell lines by nature, once established, are Immortal - therefore the development of Best-in-Class Allogeneic stem cell banks and derivative products are essential to the broad-based success of the sector.
Reprogramming Somatic Cells to Pluripotency
I was surprised also that the recent SCNT/iPS paper contradicted the previous research on the topic of somatic reprogramming genomic stability. What perhaps led to this surprise was the authorship of the paper, comprising 2 of the 3 lead scientists that had published significant SCNT results recently in support of SCNT technology and helped open up the possibility of its use once again as a therapeutic cell source.
The Fat Lady hasn't even taken the stage yet - all must be taken forward as viable possibilities until then.
Cheers
---------------------------------------------------------------------------
Pluripotent derived cells for Parkinson’s disease edging closer to clinic
As written by Malin Parmar, Associate Professor Lund University, about her team's pioneering hESC program at the University:
"A major breakthrough in the development of stem cell-derived brain cells has put researchers on a firm path towards the first ever stem cell transplantations in people with Parkinson’s disease. A new study presents the next generation of transplantable dopamine neurons produced from stem cells. These cells carry the same properties as the dopamine neurons found in the human brain."
msemporda commentary:
Parkinson's disease has been studied for decades without much clinical progress. Fetal ventral mesencephalic cell transplants have been shown to work in certain cases, while in other patients the results were inconclusive. New studies are planned by TransEuro.
Also on the horizon also are renewable sources of direct dopamine neurons derived from Pluripotent sources - hESCs, hpSCs & iPSCs (embryonic, parthenogenetic & induced pluripotent).
Other pioneers in the Parkinson space is are Jeanne Loring & Lorenz Studer. Dr. Studer who was awarded The MacArthur Fellowship in Sept 2015 as "a stem cell biologist pioneering a new method for large-scale generation of dopaminergic neurons that could provide one of the first treatments for Parkinson’s disease and prove the broader feasibility of stem cell–based therapies for other neurological disorders."
Other pioneers in the Parkinson space is are Jeanne Loring & Lorenz Studer. Dr. Studer who was awarded The MacArthur Fellowship in Sept 2015 as "a stem cell biologist pioneering a new method for large-scale generation of dopaminergic neurons that could provide one of the first treatments for Parkinson’s disease and prove the broader feasibility of stem cell–based therapies for other neurological disorders."
All pathways to facilitate an allogeneic source of cells to potentially treat Parkinson's disease will be trialed in the near future - with hpSCs looking to begin trials in 2015 in Australia.
The hope of course is that these Pluripotent sourced cells show similar to better results in their own human trials in order to further the possibilities of developing an effective treatment for the many that suffer from this and other devastating cell centric neuronal conditions.
Good reviews of the Parkinson area were published recently by EuroStemCell & Future Medicine:
Another area of stem cell development that isn't just hype but is being applied practically to a terrible disease with care and consideration.
There IS Hope & irrespective of the gallery the science is being translated. When these and other studies show these cells to be safe and effective through both FDA approved Phase I & II trials what will we say then? Wait, wait - we need many many more years of tests first... also we continue to have cell source doubt??
There comes a point when science becomes self-serving rather than the other way around. There is a reason for the designation & planning for consensual expanded/early access programs.
Cheers
Ref - ISCO hpSC Paper
---------------------------------------------------------------------------
Pluripotent Cell Sources
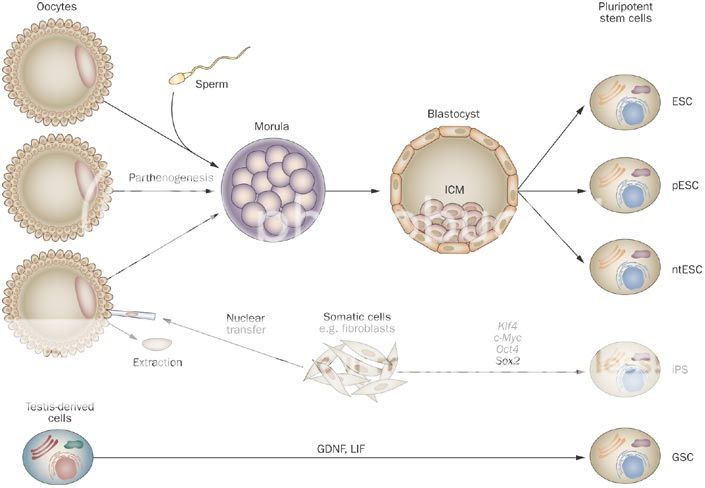
Stem cell science is inherently complex as it relates to biological systems which are only now becoming fully explored and understood. To many Stem Cell Therapeutics is new but in reality Bone Marrow (BM) transplants pioneered the sector over 40 years ago and the use of BM stem cells has been widely adopted throughout the world since then. Thus the sectors' Adult Stem Cells' origins led investigators to first develop more specific cell types for Adult Stem Cell Therapeutics, such as Adult Mesenchymal Stem Cells (MSCs) and terminal cell types of Adult MSC progenitor origin, such as Skin and Cartilage et al, which have been approved as the first purified and expanded Stem Cell Products available Internationally. To round out the background, importantly during this period James Thompson discovered a protocol to isolate, maintain and grow Human Embryonic Stem Cells (hESCs) in 1998 and the field opened up to the possibilities of using nature's master cells which are able to create any cell type in the body - i.e. a Pluripotent Cell - which Adult cells can't do. Also the potency and replicative power of these cells made hESC Therapeutics a viable cost effective medicinal option, which was therefore pursued by many in parallel to the work being done on Adult Stem Cells.
Now Pluripotent cells can be created from iPS and hESC technologies, as well as SCNT and Parthenogenesis. Adult Stem Cell researchers have also discovered recently a couple of potential sources of Pluripotent cells - Very Small Embryonic Like (VSELs) and Wisdom Tooth Dental Pulp (DPPSC) - and are investigating their capabilities. The nature of Pluripotency is important to grasp as the literature refers to Pluripotency as a general state, which also can be described as "embryonic" - however it doesn't necessarily mean derived from an Embryo, as the foregoing technologies prove. OCATA Therapeutics' (previously called Advanced Cell Technology - ACTC) historical work was to create "embryonic" cells by means of whatever technology was stable enough to generate viable Pluripotent cells... This work included extracting cells from an early stage fertilized human egg (human Embryonics - eventually Blastomere IP), from the false fertilization of a human egg, which cannot become a human (Parthenogenesis IP), from the transfer of an adult cell's nucleus to a human egg with fusion (SCNT or "Therapeutic Cloning" IP), from the reprogramming of an adult cell back in time to a Pluripotent state (De-differentiation IP - now called iPS)... Further to this OCATA was experimenting in those early days also with Trans-Differentiation, which is the reprogramming of an adult cell to directly become another adult cell type without reversing back to an intermediate Pluripotent state (Trans-differentiation IP). This early work was late 1990s to early 2000s...
As mentioned the term Pluripotency is by definition the ability to derive stem cells of any type. The Technology IP in the sector is how to create Pluripotent cells and then also how to maintain and differentiate those Pluripotent cells into the desired cell types and then scale those sufficient for Therapeutic marketing at a reasonable cost... As an analogy think of this as a Tree with a few Roots only that feed a Trunk - the Trunk is the Pluripotent state from which there emerges a number of Branches, which represent the Multipotent states, which then sprout Sub-Branches and then the leaf bearing Offshoots of those which produce the Leaves.... These Leaves are the finished individual cell types.. This is where the analogy breaks down somewhat as the Main Branches in the Tree, the Sub-Branches, the Offshoots and the Leaves would all be different colors representing different states along the unique path to the final cell types, nor does it represent the accurate number of possible intermediate step stages to get to the end result Cell Type - anyway you get the point I hope..
Human Embryonic Pluripotent Cells (hESCs) can be made by anyone soon given Thompson's WARF hESC derivation process is coming out of Patent Protection. That will open up hESC science to more innovation and programs - but it is a destructive process. In Europe the WARF Patents were cancelled and therefore the hESC research field was pursued with more interest than it was in the US. As everyone knows OCATA invented a unique approach to derive an hESC by way of a non-destructive process - Blastomere IP. That is what sets OCATA apart in getting to the Pluripotent Trunk stage using the embryo Root. No one else does that and they have a Patent in the US and Patent Applications submitted in other Territories to protect that hESC advantage. In Europe a non-destructive technology is required to pursue Patentability - OCATA awaits the European Legal process on its Blastomere Patent Application to secure its rights there. This is apart from the differentiation IP protocols of taking a Pluripotent cell and making it differentiate into a number of different Multipotent cell lineages and end cell types. These are what OCATA has a unique Priority based ability to develop and is pursuing broad Patent IP on for the Eye, Blood and Immune System areas... This positioning is unique in the world of hESC science for those areas of development IMO.
In respect to iPS Ocata are looking to secure the "Pluripotent" definition inclusion in Ocata's more recent Patent Extension Applications in all the above program areas as a right from the Original Filings and the inclusive Legal interpretation of the term "embryonic" in recent rulings. This has already been established in Australia for the Eye Program. The term iPS is a term coined by Yamanaka and as I relayed refers to taking an Adult Cell and converting it with reprogramming steps to a Pluripotent state - this is another of the Roots of the Tree Trunk. ACTC and others were doing this long before Yamanaka - OCATA's term for that was "De-differentiation" and the components of that technology are Patent Filings with v.early Priority Dates (late 1990s and early 2000s). A number of Patent Filings relate and ref to OCT4 as a differentiation / reprogramming protein used in the technological processes and methods that OCATA and others were experimenting with. In 2006 Yamanaka defined a set of 4 factors (incl. OCT4) that if used according to his protocol would convert Adult Cells back to a Pluripotent state. The fact that he documented and published this protocol in the Literature and made it widely available as a process for Lab Researchers made it a "standard" and won him the Nobel (rightly so). However, it doesn't negate the fact that other scientists had been working on the same reprogramming language, some way before Yamanaka (incl. OCATA). This is what Mgt. have been saying and that it has an early Priority on the use of OCT4 in the science. This of course relates to those using OCT4 in their protocols for iPS - however, it is an evolving field so the use of OCT4 in certain Protocols isn't necessary... I've stated my opinion on OCT4 in this iCell Thread.
Gary Rabin, then CEO of OCATA, relayed in an interview that OCATA's iPS approach was "zero-footprint reprogramming using vectorless technology" and is more to the point here as it's a reference to the difference in approaches being played out in the iPS field currently... Rather than getting caught up in defending the OCT4 Priority - which IMO Big Pharma partners will do later, if necessary - the current issue is how well do the various protocol approaches perform... That is still an open question but certainly I think most would agree now that an integration free route (i.e. not entering the genome) is safer - hence the effort to use steps that avoid integrating. However, the use of vectors (irrespective of Viral use or not) is also at issue here and it's been shown in certain cases to create residual effects. Yamanaka and Thompson use vectors. The leading safety protocols are the Harvard related iPS teams - Daley's mRNA process and Kim/OCATA's protein method... Safety first is the goal - hence the keen interest in Research Licenses for freely available integrating and non-integrating vector based iPS lines (Yamanaka and Thompson) but slow progress on Therapeutic Programs based on same... It is still too early to determine which Pluripotent route is best suited for mass market therapeutics but clearly OCATA is amongst the hand full of leaders in the field and one could say is the front runner with it's safety first iPS protocol pending IND submission selecting a denucleated cell type (Platelets) as a further safety precaution. In addition, OCATA has a potential lock on the Pluripotent RPE derivation that the other front runner, Yamanaka, is pursuing as his first indication, which would add distance to OCATA's first mover position...
In summary OCATA's Pluripotent strategy is to link this fundamental Trunk source for cell derivations lineages for the Eye, Blood and MSC Programs. Apart from that the Pluripotent protocols are valuable as stand alone Root Platforms - Blastomere, iPS Protein Reprogramming, SCNT and Parthenogenesis. These foundational Root technology approaches, plus Trans-Differentiation tech, make up the knowledge base and IP of the company generally and are interwoven as part of its Scientific Tool Kit. The breadth of the science IP protects the company from sector challenges - even if it's not using a part of the technology IP vault for a specific program.... Patents granted are more valuable than Applications of course but in this field knowledge and innovation are the keys to success given it's moving so quickly - a first mover advantage with IP is a powerful force.
To conclude it has been said that "Ocata have some of the best developmental biologists in the world" and it's clear they have IP in ALL Pluripotent technological avenues and are pioneering the science with a first mover strategy in ALL Root processes, which therefore suggests that if any one process is successfully translated this will establish OCATA's future and those associated.
Cheers
Root Pluripotent Processes:
This video below of Dr. Lanza's scientific presentation goes into detail about the various steps mentioned in the summary above - note that the Doctor talks about all Root IP approaches as part of his scientific knowledge base tool kit (hESC, SCNT, iPS and by default Parthenogenesis when referring to the Egg - see below Patent App).
The Cytoplasm is nature's incubator. What Ocata have here is a foundational approach to reprogram an old adult cell backwards in time to be youthful via a young new Egg, along the way using technology to modify.. This effectively is the road to healthy longevity via a natural cell regression mechanism that uses you're own adult cells in further steps to generate pluripotent cells for therapeutics... Previously OCATA needed access to a constant supply of human eggs and therefore it wasn't viable - now it is. SCNT is the term used for this as it stands for Somatic Cell Nuclear Transfer and is now to be combined with OCATA's iPS and Parthenogenesis Platforms using ES culturing differentiation technology for patient specific cells and tissues as an alternative route to deliver Cell Therapeutics. Genetic modifications are part of the overall process generally...
Effectively this states that OCATA uses Eggs to nurture regression, then uses SCNT technology or Parthenogenesis to derive "embryonic" cells for Therapeutic use... Before the lack of human Eggs was the bottleneck and the ethical issues associated with creating human embryos. With this technology approach we have choice and control over the entire process of developing Pluripotent cells without human fertilized embryos and without the possibility of viable embryo by products... iPS allows us to revert an Adult cell to become an Egg and with controlled stimulation "embryonic/Pluripotent" cells can be produced without any possibility of developing viable human embryos... This is Lanza's scientific stem cell tool kit brilliance and what makes OCATA unique in that it has ALL the necessary pieces of the puzzles and knows how to assemble them.
Further General References:
Embryonic stem cell - Wikipedia, the free encyclopedia
Induced pluripotent stem cell - Wikipedia, the free encyclopedia
Somatic-cell nuclear transfer - Wikipedia, the free encyclopedia
Parthenogenesis - Wikipedia, the free encyclopedia
Transdifferentiation - Wikipedia, the free encyclopedia
Adult stem cell - Wikipedia, the free encyclopedia (VSELs)
Added Dr. Zarbin's papers & presentation:
http://www.revophth.com/content/d/retina/c/37809/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176062/
http://www.asorn.org/client_data/fil...zarbin2012.pdf